Which is kind of silly, but not really...
As a member of The Short Lab, the Ecology and Evolutionary Department at KU, and the KU Biodiversity Institute, I provide updates each semester for the lab (https://sites.google.com/view/shortlab/people), department (https://eeb.ku.edu/jennifer-giron) and BI (https://biodiversity.ku.edu/entomology/people/jennifer-giron) websites. The way it usually works, is I send information to the people in charge, and they update the corresponding website for me, which can turn into annoying, both for me and the people responsible for the changes in case I forget something or find a typo later. In particular, in the case of the lab's website, it is my advisor who updates it, but he doesn't have time for my manic-obsessive nonsense, so he suggested me to create my own website, so he would only need to put a link to it and that's it.
And of course, it makes complete sense! I have administered and created websites before. Once when I was a Collection Technician at the UPRM-INVCOL in Puerto Rico (It was a Joomla-based site that does not work anymore though), and then I used Google Sites for the Ecology and Research Fundamentals classes I taught at Universidad Santo Tomás in Villavicencio, Colombia. So yeah... I have no idea why I didn't do it before.
In the creation of the website, I have realized several things:
1. It is so easy to make a website, that everyone should have one (at least everyone who wants/need to be visible). There are several advantages in getting a Google site: (1) it is free; you only need a Google account, and it can be associated with your Google Drive to upload pictures and files; (2) Is really quite simple and straightforward to create, delete and edit your website. It has a limited set of customizable templates, which also have limited style options. The good thing about this, is that (if you are like me) you spend less time thinking of something that looks good, the right combination of colors, if things are aligned, time that can be used in creating your contents.
2. It is important to have an electronic presence (at least for people that will eventually look for a job or seek collaborations). In one of the mandatory classes here at KU, the instructors, who are professors often actively looking for potential students, technicians and postdocs, told us that every time there is a suitable candidate, they look the person up on the internet, and it is usually taken as a good sign if the person is not a ghost. As part of the class, I got an ORCID and set up my Google Scholar profile. What better way to showcase your research than your own website??
3. There are so many things I have done that are nowhere but on my computer (not even in my CV) and it would be nice to share them with the world. Sometimes I have needed to access certain information and it is only in a book at my lab's desk for example. That's why I put a list on my website of the genera currently assigned to the subfamily Acidocerinae, and a list of the known species of acidocerines of the New World. I have also tried to add references with links to the ones findable online, so that would save some time to future me or my readers.
There are also scattered (and sort of temporary) efforts like my blogs (Entiminae and Acidocerinae) and a Project Noah account, that would be nice to have tied directly to me somehow.
4. You can share as much information as you want, presented in the way you want to show it (without the usual constraints of institutional websites). It also allows you to be more personal than if someone simply goes through your CV. You can tell stories, put pictures, give more details about what you do. This for me has one good thing: it may serve as a "passive science communication": you are not directly speaking to people (which has proven challenging for me, worse if not in my native language), but you are sharing your experiences by showing how you do science. You may be giving people ideas or encouragement, even for just to reach out to you for help.
5. You can also add information on your side projects. Sometimes people would tell me about this awesome project that a friend/colleague of them was doing but never published because you know, life happens. In your own website you can have space for raw "products" that might be useful for someone else, in terms of methods at least. You already spent time on it, better put those to a good use by giving them the potential to be inspiring.
So here is the link: https://sites.google.com/view/jcgiron/home
As with most things, it does come with a price: you will have to keep your website updated! in this context, there is nothing sadder than outdated websites... occasionally I have found really cool blogs, but you can tell the project died or got abandoned at some point (actually I guess that might be the impression from my entimine blog :'( ), and then the owner is nowhere to be found on the web. If your projects (even if unfinished) can be found somewhere, you will probably shed some light on someone's path, or at least you can feel a little famous when someone contacts you because of your website!
Acidocerinae
Friday, March 23, 2018
Thursday, December 21, 2017
Closer to the molecules
It's been a while since my last post... again!
This semester was full of many things going on: classes, comprehensive exam, ESA meeting, work (in two publications -mostly one- that are not part of my dissertation), finishing one weevil paper (of my queue of 3; nothing to do with dissertation either).
One of the classes I took this semester was Phylogenetic Methods with Dr. Mark Holder at the EEB Department at KU.
Overall the course was very well planned and executed. I just kind of wish I have had better computational skills, and that I was less stubborn to begin with. Just after our first two lab sessions I realized I'm strongly attached to WinClada (perhaps by learned habit), not only because (in my opinion) it makes creating/editing matrices way simpler than Mesquite (don't get me wrong, I like Mesquite. It can do many more things than WinClada), but also because I had not used (was probably avoiding) other programs to perform phylogenetic analyses. So far I had only worked with morphological data, and refused to leave my parsimonious comfort zone. After trying the GUI version of PAUP*, I was sold!
Then, when we started using Maximum Likelihood (ML) and Bayesian inference programs, it was really challenging for me to get all the command-line applications to work, and at the same time satisfying when the programs finally ran. Also because, as I mentioned before, I'm stubborn and always try to figure things out by myself, instead of asking for help, which is just time-consuming.
The last task for the class was to apply some of the tools we learned to a dataset, so I performed ML and Bayesian phylogenetic analyses to the current dataset that we have for the Acidocerinae (170 terminals + 11 outgroups). For most of those terminals we have sequence alignments for the mitochondrial loci COI, and the nuclear loci 18S, 28S, H3, and CAD.
For the concatenated dataset the results are highly consistent between methods (and roughly in agreement with the molecular analysis presented by Short & Fikáček (2013)). For the most part the same groupings were recovered with comparable support. Given that some of the clades obtained were not well supported (see the grey box in the figure on the right), I also made ML analyses for each of the genes to check for gene-tree discordance. It was very interesting to see how each gene tells a different story, and then how the tale that each particular gene tells, is very similar to previous classification schemes proposed for what now is known as the Acidocerinae. No wonder why this group of hydrophilids is so confusing!
As a point of information, the clades in the grey box are exclusively Neotropical, and only fairly recently discovered. Some of them have been described in the past 20 years or so, and some are only now being described (although present in collections since the 60's - 80's).
The clade highlighted in orange corresponds to Quadriops, the only known terrestrial acidocerine, that we recently revised. You can find the publication here: https://zookeys.pensoft.net/article/19815/
The clade in green corresponds to Globulosis, whose revision also came out this year: https://biotaxa.org/Zootaxa/article/view/zootaxa.4232.2.10
The clade in purple (at least the Tobochares part of it) was also revised by members of our lab this year: https://zookeys.pensoft.net/article/11773/
So all in all, I feel satisfied with the semester (and glad it is finally over!!). The exercise with the molecular dataset was very rewarding for me, and a good way to make me more comfortable working with molecular data and the many parameters you have to keep in mind, and the tons of resources (people among those) that are available for you to make better decisions and obtain more robust phylogenies. Also, it would be interesting to have more genes (more stories) to add to the mix, but then again, we need a stop-point along the way.
References
Girón, J.C. & Short, A.E.Z. (2017) Revision of the Neotropical water scavenger beetle genus Quadriops Hansen, 1999 (Coleoptera, Hydrophilidae, Acidocerinae). ZooKeys 705: 115–141.
Short, A.E.Z., García, M., Girón, J.C. (2017) Revision of the Neotropical water scavenger beetle genus Globulosis García, 2001 (Coleoptera: Hydrophilidae: Acidocerinae). Zootaxa 4232(2): 271–281.
This semester was full of many things going on: classes, comprehensive exam, ESA meeting, work (in two publications -mostly one- that are not part of my dissertation), finishing one weevil paper (of my queue of 3; nothing to do with dissertation either).
One of the classes I took this semester was Phylogenetic Methods with Dr. Mark Holder at the EEB Department at KU.
 |
| Maximum Likelihood phylogeny for part of the Acidocerinae |
Then, when we started using Maximum Likelihood (ML) and Bayesian inference programs, it was really challenging for me to get all the command-line applications to work, and at the same time satisfying when the programs finally ran. Also because, as I mentioned before, I'm stubborn and always try to figure things out by myself, instead of asking for help, which is just time-consuming.
The last task for the class was to apply some of the tools we learned to a dataset, so I performed ML and Bayesian phylogenetic analyses to the current dataset that we have for the Acidocerinae (170 terminals + 11 outgroups). For most of those terminals we have sequence alignments for the mitochondrial loci COI, and the nuclear loci 18S, 28S, H3, and CAD.
For the concatenated dataset the results are highly consistent between methods (and roughly in agreement with the molecular analysis presented by Short & Fikáček (2013)). For the most part the same groupings were recovered with comparable support. Given that some of the clades obtained were not well supported (see the grey box in the figure on the right), I also made ML analyses for each of the genes to check for gene-tree discordance. It was very interesting to see how each gene tells a different story, and then how the tale that each particular gene tells, is very similar to previous classification schemes proposed for what now is known as the Acidocerinae. No wonder why this group of hydrophilids is so confusing!
As a point of information, the clades in the grey box are exclusively Neotropical, and only fairly recently discovered. Some of them have been described in the past 20 years or so, and some are only now being described (although present in collections since the 60's - 80's).
The clade highlighted in orange corresponds to Quadriops, the only known terrestrial acidocerine, that we recently revised. You can find the publication here: https://zookeys.pensoft.net/article/19815/
The clade in green corresponds to Globulosis, whose revision also came out this year: https://biotaxa.org/Zootaxa/article/view/zootaxa.4232.2.10
The clade in purple (at least the Tobochares part of it) was also revised by members of our lab this year: https://zookeys.pensoft.net/article/11773/
So all in all, I feel satisfied with the semester (and glad it is finally over!!). The exercise with the molecular dataset was very rewarding for me, and a good way to make me more comfortable working with molecular data and the many parameters you have to keep in mind, and the tons of resources (people among those) that are available for you to make better decisions and obtain more robust phylogenies. Also, it would be interesting to have more genes (more stories) to add to the mix, but then again, we need a stop-point along the way.
References
Girón, J.C. & Short, A.E.Z. (2017) Revision of the Neotropical water scavenger beetle genus Quadriops Hansen, 1999 (Coleoptera, Hydrophilidae, Acidocerinae). ZooKeys 705: 115–141.
Kohlenberg, A.T. & Short, A.E.Z. (2017) Revision of the Neotropical water scavenger beetle genus Tobochares Short & García, 2007 (Coleoptera: Hydrophilidae: Acidocerinae). ZooKeys 669: 113–146.
Short, A.E.Z. & Fikáček, M. (2013) Molecular Phylogeny, Evolution, and Classification of the Hydrophilidae(Coleoptera). Systematic Entomology 38: 723–752.
Short, A.E.Z. & Fikáček, M. (2013) Molecular Phylogeny, Evolution, and Classification of the Hydrophilidae(Coleoptera). Systematic Entomology 38: 723–752.
Short, A.E.Z., García, M., Girón, J.C. (2017) Revision of the Neotropical water scavenger beetle genus Globulosis García, 2001 (Coleoptera: Hydrophilidae: Acidocerinae). Zootaxa 4232(2): 271–281.
Monday, September 4, 2017
so... where to stop?
This has happened to me before... once you start looking at things, you keep finding more, and more, and more structures, characters and character states, and then you really don't know where to stop... maybe it has to do with my obsessive-compulsive behavior.
At this point for me, there are two main causes of a need-to-stop decision: one has to do with the taxonomy, the other with the anatomy.
As you may know, I've been looking at the morphology of the Hydrophilidae and lately have been focused on head and mouthparts.
On the taxonomic side, a need-to-stop comes from getting too excited in your task to find the origin of your characters. There are some structures that are consistent across an entire family, and when you compare to works in other related groups, the same structure does not look at all as what you see in your group of interest. It could be a synapomorphy, but how to be sure?. Then you keep going up the tree to end up looking at other related families. If the same character state is present there, the next logical step would be to keep going up the tree. And then you end up looking at a very large group that not necessarily answers what you were asking, but leaves you with even more questions. So where should you stop? because at some point this can become an avalanche of characters and character states that can easily end up in a never ending story. Fortunately, advisors exist and get you back to Earth, limiting the outgroup sampling so you don't go (too) crazy [they -usually- know better].
An example of this would be the hypopharyngeal-premental sclerites which are conserved across hydrophilids, in contrast with what has been described by Weide et. al (2014) for Aleocharine staphilinids, and also VERY different from what you see in Scarabaeinae (see for example Tarasov & Génier 2015). It is a very tridimensional structure formed by fusions of the hypopharyngeal suspensoria (see Snodgrass 1935). Question is, where did this particular shape originated?.
As for the anatomic side of a need-to-stop situation, there is this fine example: the maxillae and the mentum were studied by Williams in 1938 across Coleoptera. She described and illustrated these structures for Tropisternus glaber. The problem is that when you compare her drawings to a real sample, it looks like this:
As you can see, there are way more characters to look at, and it gets "worse" when you start looking at other genera, as more character states become more apparent.
So again, where do you stop among so many details? you can easily spend a lifetime just looking at each varying component of these structures (and I'm not even considering musculature, for example, and I'm only using one sample preparation technique). Then I guess it is a matter of judgement, practicality, informational value, and being conscious that you don't have a lifetime to complete your dissertation!
References
Tarasov, S. & Génier, F. (2015) Innovative bayesian and parsimony phylogeny of dung beetles (Coleoptera, Scarabaeidae, Scarabaeinae) enhanced by ontology-based partitioning of morphological characters. PLoS ONE 10(3): e0116671.
Weide, D., Thayer, M. K., & Betz, O. (2014). Comparative morphology of the tentorium and hypopharyngeal–premental sclerites in sporophagous and non‐sporophagous adult Aleocharinae (Coleoptera: Staphylinidae). Acta Zoologica, 95(1), 84-110.
Williams, I. W. (1938). The comparative morphology of the mouthparts of the order Coleoptera treated from the stand-point of phylogeny. Journal of the New York Entomological Society, 46(3), 245-289.
At this point for me, there are two main causes of a need-to-stop decision: one has to do with the taxonomy, the other with the anatomy.
As you may know, I've been looking at the morphology of the Hydrophilidae and lately have been focused on head and mouthparts.
On the taxonomic side, a need-to-stop comes from getting too excited in your task to find the origin of your characters. There are some structures that are consistent across an entire family, and when you compare to works in other related groups, the same structure does not look at all as what you see in your group of interest. It could be a synapomorphy, but how to be sure?. Then you keep going up the tree to end up looking at other related families. If the same character state is present there, the next logical step would be to keep going up the tree. And then you end up looking at a very large group that not necessarily answers what you were asking, but leaves you with even more questions. So where should you stop? because at some point this can become an avalanche of characters and character states that can easily end up in a never ending story. Fortunately, advisors exist and get you back to Earth, limiting the outgroup sampling so you don't go (too) crazy [they -usually- know better].
An example of this would be the hypopharyngeal-premental sclerites which are conserved across hydrophilids, in contrast with what has been described by Weide et. al (2014) for Aleocharine staphilinids, and also VERY different from what you see in Scarabaeinae (see for example Tarasov & Génier 2015). It is a very tridimensional structure formed by fusions of the hypopharyngeal suspensoria (see Snodgrass 1935). Question is, where did this particular shape originated?.
 |
| Mentum in lateral view: staphilinid, dung beetle and Globulosis |
 |
| Maxilla (top), Mentum (bottom): Tropisternus glaber (by Williams 1938); Globulosis hemisphericus. |
As you can see, there are way more characters to look at, and it gets "worse" when you start looking at other genera, as more character states become more apparent.
 |
| Maxilla, different groups of hydrophilids. |
So again, where do you stop among so many details? you can easily spend a lifetime just looking at each varying component of these structures (and I'm not even considering musculature, for example, and I'm only using one sample preparation technique). Then I guess it is a matter of judgement, practicality, informational value, and being conscious that you don't have a lifetime to complete your dissertation!
References
Tarasov, S. & Génier, F. (2015) Innovative bayesian and parsimony phylogeny of dung beetles (Coleoptera, Scarabaeidae, Scarabaeinae) enhanced by ontology-based partitioning of morphological characters. PLoS ONE 10(3): e0116671.
Weide, D., Thayer, M. K., & Betz, O. (2014). Comparative morphology of the tentorium and hypopharyngeal–premental sclerites in sporophagous and non‐sporophagous adult Aleocharinae (Coleoptera: Staphylinidae). Acta Zoologica, 95(1), 84-110.
Williams, I. W. (1938). The comparative morphology of the mouthparts of the order Coleoptera treated from the stand-point of phylogeny. Journal of the New York Entomological Society, 46(3), 245-289.
Friday, April 21, 2017
I'm on my way
So the last time I wrote a post here I was basically complaining about the lack of information regarding detailed morphologies. Most papers I found at the time concerning mouthparts, for example, would be limited to external or just to the general structures, obviating the details.
I kept looking and I have found some impressive works in Staphylinidae, Scarabaeinae and Leiodidae, all of them groups fairly close to the Hydrophilidae, that have illuminated the path for me to understand what I'm seeing.
Betz, Thayer & Newton in 2003 published a very thorough survey of the mouthparts of sporophagous staphylinids. For the most part, they used Scanning Electron Microscopy (SEM) in order to illustrate the different kinds of variations in the components of the mouthparts, and included descriptions of their main functional elements, as well as a discussion on the structural and functional requirements for different feeding habits.
In 2014, Weide, Thayer & Betz published a comparative work on the morphology of the tentorium and hypopharyngeal–premental sclerites in aleocharine staphilinids. This paper contains amazing 3D reconstructions of micro-CT scan generated images of the heads of 19 different species. The images help a lot in understanding the relative positions of structures inside the head. In all the images, the structures that are referred in the descriptions are labeled, which is extremely helpful.
Another valuable source of information was produced by Tarasov & Génier in 2015 for dung beetles. They present photographs taken through a microscope, indicating and briefly describing the structures they used in their phylogenetic analysis. Another very interesting contribution of this paper is that they use what they call an ontology approach, in order to generate partitions of the morphological data, which will come in handy for me in the future.
There is also a paper by Antunes-Carvalho et. al (2016), which discuss the cephalic anatomy of a leiodid species. They present photographs of the entire head, SEM images of the head, antennae and mouthparts, and micro-CT scan reconstructions of the tentorium, including tissue associated to the nervous system and musculature. The illustrations and discussions presented in this paper provide some sort of template for what can (should?) be noted in beetles.
By going through the information presented in these papers and looking at my specimens, I have come to several conclusions and resolutions. Regarding the imaging method, each has advantages and disadvantages:
- Regular photographs through a microscope work very well, but are problematic when the structures have certain volume and/or overlapping parts and/or lots of setae, because when you stack a series of photos, the resulting image loses resolution in terms of the structures that you can see. The advantage is that as your structures are likely floating in glycerin, you can easily look at them in the scope and move them around as you please, and the issue of them sailing away when you are trying to photograph them, is easily solved using denser solutions as hand sanitizer or KY gel.
- SEM images are amazing for recognizing areas with different surfaces, different kinds of setae, distinguish arrangements of those structures, but sometimes you cannot tell apart sclerotized areas from membranes, let alone the concealing of internal structures. Other drawbacks include that the required drying process can distort the samples, and then mounting tiny pieces in SEM stubs is probably tricky and once the sample touches the sticky surface, there is no way to re-accommodate without ripping off things. So basically you should think carefully what view is the most informative, which is a straightforward decision with fairly flat structures, getting complicated with bulkiness. Another issue has to do with the cleanliness of the sample; sometimes structures get obscured by a layer of whatever the specimen was eating when it was collected. This issue can be easily solved with large specimens/structures, but is almost impossible with too tiny samples.
- Using micro-CT scan would be great! I mean, that's the dream! but the availability of the equipment for that is still very limited (and probably expensive); also, it seems to me that assembling and interpreting the resulting reconstructions takes time (I guess until you get used to do it). Advantages of this method have to do with the fact that is a non-destructive method that only requires specimens to be dry to the critical point; so you can even study old holotypes without even touch them, which is pretty convenient.
The fact that different works have employed different imaging techniques is helpful, but somehow it adds another level of complexity, given that making comparisons gets tricky, not only because you are comparing the same structure portrayed using different methods, but also because you are comparing taxa that even being fairly close, exhibit striking differences.
Even with the accuracy of the imaging techniques used across works, it is definitely so much better to look at the structures directly!! Don't get me wrong, authors really make an effort to show precisely what they see, but is not the same to look at a nice picture, than to be able to see the structures in the actual specimens and say "aha! it is exactly like in the picture!" It happened to me with the labrum of the Scarabaeinae as illustrated by López-Guerrero & Zunino (2007): only when I saw it in the specimen I realized how sort of reduced and highly specialized the structure is!
Here is an example of the mentum and all its wonders, indicating one of its associated sclerites, as viewed in an staphilinid, a dung beetle and Globulosis... all strikingly different from each other!!!
It has also helped that now, for the most part, I'm more careful and patient when dissecting, so I get to see what is attached to, or in contact with what.
At this time I think the best approach would be to name and describe everything I see, noting how they compare to works in other groups, so if anyone gets to check on those structures in the future, she will know where to look. I guess trying to establish homologies at this point, without a developmental basis, could be misleading (even if "only" implied by the use of names in common).
On a last note, I really wish I had a colleague to discuss this things with...
References
I kept looking and I have found some impressive works in Staphylinidae, Scarabaeinae and Leiodidae, all of them groups fairly close to the Hydrophilidae, that have illuminated the path for me to understand what I'm seeing.
Betz, Thayer & Newton in 2003 published a very thorough survey of the mouthparts of sporophagous staphylinids. For the most part, they used Scanning Electron Microscopy (SEM) in order to illustrate the different kinds of variations in the components of the mouthparts, and included descriptions of their main functional elements, as well as a discussion on the structural and functional requirements for different feeding habits.
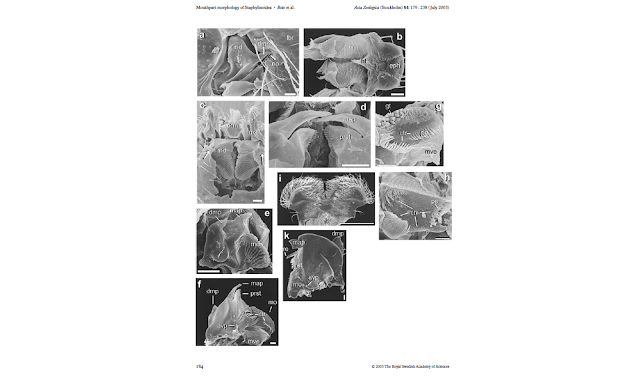 | |
|
 |
| Weide et al 2014, part of figure 3 |
 |
| Tarasov & Génier 2016, fig 42. |
 |
| Antunes-Carvalho et al 2016, figures 3, 5 and 1 respectively. |
- Regular photographs through a microscope work very well, but are problematic when the structures have certain volume and/or overlapping parts and/or lots of setae, because when you stack a series of photos, the resulting image loses resolution in terms of the structures that you can see. The advantage is that as your structures are likely floating in glycerin, you can easily look at them in the scope and move them around as you please, and the issue of them sailing away when you are trying to photograph them, is easily solved using denser solutions as hand sanitizer or KY gel.
- SEM images are amazing for recognizing areas with different surfaces, different kinds of setae, distinguish arrangements of those structures, but sometimes you cannot tell apart sclerotized areas from membranes, let alone the concealing of internal structures. Other drawbacks include that the required drying process can distort the samples, and then mounting tiny pieces in SEM stubs is probably tricky and once the sample touches the sticky surface, there is no way to re-accommodate without ripping off things. So basically you should think carefully what view is the most informative, which is a straightforward decision with fairly flat structures, getting complicated with bulkiness. Another issue has to do with the cleanliness of the sample; sometimes structures get obscured by a layer of whatever the specimen was eating when it was collected. This issue can be easily solved with large specimens/structures, but is almost impossible with too tiny samples.
- Using micro-CT scan would be great! I mean, that's the dream! but the availability of the equipment for that is still very limited (and probably expensive); also, it seems to me that assembling and interpreting the resulting reconstructions takes time (I guess until you get used to do it). Advantages of this method have to do with the fact that is a non-destructive method that only requires specimens to be dry to the critical point; so you can even study old holotypes without even touch them, which is pretty convenient.
The fact that different works have employed different imaging techniques is helpful, but somehow it adds another level of complexity, given that making comparisons gets tricky, not only because you are comparing the same structure portrayed using different methods, but also because you are comparing taxa that even being fairly close, exhibit striking differences.
Even with the accuracy of the imaging techniques used across works, it is definitely so much better to look at the structures directly!! Don't get me wrong, authors really make an effort to show precisely what they see, but is not the same to look at a nice picture, than to be able to see the structures in the actual specimens and say "aha! it is exactly like in the picture!" It happened to me with the labrum of the Scarabaeinae as illustrated by López-Guerrero & Zunino (2007): only when I saw it in the specimen I realized how sort of reduced and highly specialized the structure is!
Here is an example of the mentum and all its wonders, indicating one of its associated sclerites, as viewed in an staphilinid, a dung beetle and Globulosis... all strikingly different from each other!!!
At this time I think the best approach would be to name and describe everything I see, noting how they compare to works in other groups, so if anyone gets to check on those structures in the future, she will know where to look. I guess trying to establish homologies at this point, without a developmental basis, could be misleading (even if "only" implied by the use of names in common).
On a last note, I really wish I had a colleague to discuss this things with...
References
- Antunes-Carvalho, C., Yavorskaya, M., Gnaspini, P., Ribera, I., Hammel, J. U., Beutel, R. G. (2017). Cephalic anatomy and three-dimensional reconstruction of the head of Catops ventricosus (Weise, 1877) (Coleoptera: Leiodidae: Cholevinae). Organisms Diversity & Evolution, 17: 199.
- Betz, O., Thayer, M. K., & Newton, A. F. (2003). Comparative morphology and evolutionary pathways of the mouthparts in spore‐feeding Staphylinoidea (Coleoptera). Acta Zoologica, 84(3), 179-238.
- López-Guerrero, I. & Zunino, M. (2007). Consideraciones acerca de la evolución de las piezas bucales en los Onthophagini (Coleoptera: Scarabaeidae) en relación con diferentes regímenes alimenticios. Interciencia, 32(7), 482-489.
- Tarasov, S. & Génier, F. (2015) Innovative bayesian and parsimony phylogeny of dung beetles (Coleoptera, Scarabaeidae, Scarabaeinae) enhanced by ontology-based partitioning of morphological characters. PLoS ONE 10(3): e0116671.
- Weide, D., Thayer, M. K., & Betz, O. (2014). Comparative morphology of the tentorium and hypopharyngeal–premental sclerites in sporophagous and non‐sporophagous adult Aleocharinae (Coleoptera: Staphylinidae). Acta Zoologica, 95(1), 84-110.
Thursday, October 27, 2016
What am I missing?
I guess this post is some sort of a ranting, but also a call for help if anyone can point me on the right direction.
Let me be clear that this is my opinion which has arisen from looking at several publications at different levels of specificity, where for me, there is not enough detail (compared to what I'm seeing on my specimen) or very precise representations (again, my opinion).
I'm working on the description of the mouthparts of one species of Globulosis (Hydrophilidae: Acidocerinae) and today I'm feeling a little bit frustrated (probably accumulated frustration is finally escaping), and several reasons come to mind, specifically regarding publications and available information. I've checked general publications for Coleoptera, for insect morphology as well as specific publications on Hydrophilids (and relatives),
Australian Beetles (2013) by Lawrence and Slipinski do a very good job at listing thoroughly and describing general structures of the adult morphology. They include many neat images, but then, there are no labels for specific structures in some of the plates (I realize this might sound picky, but labels reduce ambiguity), and fair enough, if you are into beetle morphology you are supposed to know which sclerite corresponds to which structure, or at least you for sure know Snodgrass (1993).
Snodgrass (1993) is a more general publication in taxonomic scope, but far much detailed on the morphological treatment. It includes for example names for the areas of a mandible, details on the musculature and somehow an evolutionary perspective on what is the ancestral condition and what are some of the particular variations on some orders. It also includes very detailed and fully labeled illustrations. Extremely helpful, but then, as it includes variation among orders (and not within, which is completely fine for this kind of work), it results complicated for me to figure out what is it that I'm looking at, specifically with the whole epipharynx, cibarium, hypopharynx, also because in the specimen I'm looking at, there are several other sclerites and membranous areas that I'm not sure how to name.
There is another nice (but very general) publication on mouthparts of Coleoptera from Williams (1938), with labeled illustrations, but it only includes the maxilla and the ventral side of the labium.
So I turned to publications on Hydrophilids and relatives... for one thing, most of the descriptions and revisions only refer to what you see externally, which is kind of straightforward and it is completely fine (until you become obsessed with morphological features and variation as I think I am) . A few works, compared to the amount of descriptions/revisions out there that I have seen so far, nicely illustrate dissected mouthparts, for example Hansen (1991) for Hydrophiloidea, Anton & Beutel (2004) for Helophorus, Komarek (2004) for Anacaena, Fikáček & Vondráček (2014) for Pseudorygmodus. My problem with these has more to do with the descriptive part rather than with the illustrations (don't get too excited, I also have issues with some illustrations), as some authors are more thorough than others, and then there is no consistency among publications that allow comparison.
There is an additional attempt to describe mouthparts of an hydrophilid by Shukla & Upadhyý (1978), but I have mixed opinions on that one, so no further comments in here.
In addition, there are some very impressive works by Arens (1989, 1994) on mouthparts of aquatic invertebrates as cases of convergence.
My whole point with all this complaining is that when I look at the mouthparts of this tiny brown creature, there is so so much amazement... so many details, so much perfection and craziness at the same time, that I can't help but think why they don't even look at this structures??? they are not boring at all!! would they be too complicated for people to dissect and describe? In my experience with weevils, mouthparts might not be very informative at species level, but they certainly are at genus level and higher, so why just avoid/ignore them? is it because it takes longer to prepare, dissect and describe mouthparts than simply squeeze molecular sequences out of the specimens?
Here is the tiny and mind-blowing mentum of Globulosis in lateral view... the one I haven't finished figuring out.
There are many reasons for me to be fascinated by mouthparts, one of those is what they say "you are what you eat". Wouldn't it be awesome to learn what and how are this things eating? I would love to have the Ant-Man suit and go live on a stream or a seepage to see what this guys are up to at dinner time!
And then a sense of envy comes to me from two main sources:
- The Hymenoptera Anatomy Ontology Portal, where lucky hymenopterists have an awesome resource to reach when they get lost in morphology and terminology usage, including references and images that point exactly what they are referring to. I know that a few years ago there was (?) an initiative to build the Coleoptera Anatomy Ontology, but there is not available information on that.
- There are several works on comparative morphology of mouthparts in Staphylinidae, Chrysomelidae and Curculionidae. Even Diptera people have looked at this things!
I'm aware that I might be asking for too much detail in publications, but there are works out there, just not what I want and need to see. Perhaps (and hopefully?) it is my duty to fill in this gap on the knowledge of Hydrophilids... let's see.
References
Anton, E., & Beutel, R. G. (2004). On the head morphology and systematic position of Helophorus (Coleoptera: Hydrophiloidea: Helophoridae). Zoologischer Anzeiger-A Journal of Comparative Zoology, 242(4), 313-346.
Arens, W. (1989). Comparative functional morphology of the mouthparts of stream animals feeding on epilithic algae. Archiv für Hydrobiologie. Supplementband. Monographische Beiträge, 83(3), 253-354.
Arens, W. (1994). Striking convergence in the mouthpart evolution of stream‐living algae grazers. Journal of Zoological Systematics and Evolutionary Research, 32(4), 319-343.
Fikáček, M., & Vondráček, D. (2014). A review of Pseudorygmodus (Coleoptera: Hydrophilidae), with notes on the classification of the Anacaenini and on distribution of genera endemic to southern South America. Acta Entomologica Musei Nationalis Pragae, 54(2), 479-514.
Hansen, M. (1991). The hydrophiloid beetles. Phylogeny, classification and a revision of the genera (Coleoptera: Hydrophilidae). Biologiske Skrifter, 40, 1–367.
Komarek, A. (2004) Taxonomic revision of Anacaena Thomson, 1859 I. Afrotropical species. Koleopterologische Rundschau, 74, 303-349.
Lawrence, J., & Slipinski, A. (2013). Australian Beetles Volume 1: Morphology, Classification and Keys (Vol. 1). CSIRO PUBLISHING.
Shukla, G. S., & Upadhyý, V. B. (1978). Studies on the morphology of mouth parts of Dactylosternum hydrophilioides (Coleoptera, Hydrophilidae). Deutsche Entomologische Zeitschrift, 25(1‐3), 63-69.
Snodgrass, R. E. (1993). Principles of Insect Morphology, with a new foreword by George C. Eickwort. (Cornell University Press: Ithaca, NY.) 667 pp.
Williams, I. W. (1938). The comparative morphology of the mouthparts of the order Coleoptera treated from the stand-point of phylogeny. Journal of the New York Entomological Society, 46(3), 245-289.
Let me be clear that this is my opinion which has arisen from looking at several publications at different levels of specificity, where for me, there is not enough detail (compared to what I'm seeing on my specimen) or very precise representations (again, my opinion).
I'm working on the description of the mouthparts of one species of Globulosis (Hydrophilidae: Acidocerinae) and today I'm feeling a little bit frustrated (probably accumulated frustration is finally escaping), and several reasons come to mind, specifically regarding publications and available information. I've checked general publications for Coleoptera, for insect morphology as well as specific publications on Hydrophilids (and relatives),
Australian Beetles (2013) by Lawrence and Slipinski do a very good job at listing thoroughly and describing general structures of the adult morphology. They include many neat images, but then, there are no labels for specific structures in some of the plates (I realize this might sound picky, but labels reduce ambiguity), and fair enough, if you are into beetle morphology you are supposed to know which sclerite corresponds to which structure, or at least you for sure know Snodgrass (1993).
Snodgrass (1993) is a more general publication in taxonomic scope, but far much detailed on the morphological treatment. It includes for example names for the areas of a mandible, details on the musculature and somehow an evolutionary perspective on what is the ancestral condition and what are some of the particular variations on some orders. It also includes very detailed and fully labeled illustrations. Extremely helpful, but then, as it includes variation among orders (and not within, which is completely fine for this kind of work), it results complicated for me to figure out what is it that I'm looking at, specifically with the whole epipharynx, cibarium, hypopharynx, also because in the specimen I'm looking at, there are several other sclerites and membranous areas that I'm not sure how to name.
There is another nice (but very general) publication on mouthparts of Coleoptera from Williams (1938), with labeled illustrations, but it only includes the maxilla and the ventral side of the labium.
So I turned to publications on Hydrophilids and relatives... for one thing, most of the descriptions and revisions only refer to what you see externally, which is kind of straightforward and it is completely fine (until you become obsessed with morphological features and variation as I think I am) . A few works, compared to the amount of descriptions/revisions out there that I have seen so far, nicely illustrate dissected mouthparts, for example Hansen (1991) for Hydrophiloidea, Anton & Beutel (2004) for Helophorus, Komarek (2004) for Anacaena, Fikáček & Vondráček (2014) for Pseudorygmodus. My problem with these has more to do with the descriptive part rather than with the illustrations (don't get too excited, I also have issues with some illustrations), as some authors are more thorough than others, and then there is no consistency among publications that allow comparison.
There is an additional attempt to describe mouthparts of an hydrophilid by Shukla & Upadhyý (1978), but I have mixed opinions on that one, so no further comments in here.
In addition, there are some very impressive works by Arens (1989, 1994) on mouthparts of aquatic invertebrates as cases of convergence.
My whole point with all this complaining is that when I look at the mouthparts of this tiny brown creature, there is so so much amazement... so many details, so much perfection and craziness at the same time, that I can't help but think why they don't even look at this structures??? they are not boring at all!! would they be too complicated for people to dissect and describe? In my experience with weevils, mouthparts might not be very informative at species level, but they certainly are at genus level and higher, so why just avoid/ignore them? is it because it takes longer to prepare, dissect and describe mouthparts than simply squeeze molecular sequences out of the specimens?
Here is the tiny and mind-blowing mentum of Globulosis in lateral view... the one I haven't finished figuring out.
There are many reasons for me to be fascinated by mouthparts, one of those is what they say "you are what you eat". Wouldn't it be awesome to learn what and how are this things eating? I would love to have the Ant-Man suit and go live on a stream or a seepage to see what this guys are up to at dinner time!
And then a sense of envy comes to me from two main sources:
- The Hymenoptera Anatomy Ontology Portal, where lucky hymenopterists have an awesome resource to reach when they get lost in morphology and terminology usage, including references and images that point exactly what they are referring to. I know that a few years ago there was (?) an initiative to build the Coleoptera Anatomy Ontology, but there is not available information on that.
- There are several works on comparative morphology of mouthparts in Staphylinidae, Chrysomelidae and Curculionidae. Even Diptera people have looked at this things!
I'm aware that I might be asking for too much detail in publications, but there are works out there, just not what I want and need to see. Perhaps (and hopefully?) it is my duty to fill in this gap on the knowledge of Hydrophilids... let's see.
References
Anton, E., & Beutel, R. G. (2004). On the head morphology and systematic position of Helophorus (Coleoptera: Hydrophiloidea: Helophoridae). Zoologischer Anzeiger-A Journal of Comparative Zoology, 242(4), 313-346.
Arens, W. (1989). Comparative functional morphology of the mouthparts of stream animals feeding on epilithic algae. Archiv für Hydrobiologie. Supplementband. Monographische Beiträge, 83(3), 253-354.
Arens, W. (1994). Striking convergence in the mouthpart evolution of stream‐living algae grazers. Journal of Zoological Systematics and Evolutionary Research, 32(4), 319-343.
Fikáček, M., & Vondráček, D. (2014). A review of Pseudorygmodus (Coleoptera: Hydrophilidae), with notes on the classification of the Anacaenini and on distribution of genera endemic to southern South America. Acta Entomologica Musei Nationalis Pragae, 54(2), 479-514.
Hansen, M. (1991). The hydrophiloid beetles. Phylogeny, classification and a revision of the genera (Coleoptera: Hydrophilidae). Biologiske Skrifter, 40, 1–367.
Komarek, A. (2004) Taxonomic revision of Anacaena Thomson, 1859 I. Afrotropical species. Koleopterologische Rundschau, 74, 303-349.
Lawrence, J., & Slipinski, A. (2013). Australian Beetles Volume 1: Morphology, Classification and Keys (Vol. 1). CSIRO PUBLISHING.
Shukla, G. S., & Upadhyý, V. B. (1978). Studies on the morphology of mouth parts of Dactylosternum hydrophilioides (Coleoptera, Hydrophilidae). Deutsche Entomologische Zeitschrift, 25(1‐3), 63-69.
Snodgrass, R. E. (1993). Principles of Insect Morphology, with a new foreword by George C. Eickwort. (Cornell University Press: Ithaca, NY.) 667 pp.
Williams, I. W. (1938). The comparative morphology of the mouthparts of the order Coleoptera treated from the stand-point of phylogeny. Journal of the New York Entomological Society, 46(3), 245-289.
Wednesday, October 5, 2016
In preparing and presenting at the ICE 2016
It is always a challenge to prepare presentations for scientific meetings. You always want to do a good job in effectively communicating your research, in a way that you don't confuse or bore people, which becomes trickier when you are new to the group you are working with and even more defiant when your audience is composed by people that has been working on this for many years and are recognized experts in the field.
Thing is that I'm more interested and, so far, much more focused on morphology, which was only one of the three main themes I was supposed to talk about. I consider myself a lucky person in general. I've had all the support, data and information I've needed from my advisor, Dr. Andrew Short, our postdoc researcher, Dr. Emmanuel Toussaint and my labmates at the Short Lab. It was not easy to get smooth transitions between the molecular phylogeny that we have now, the different ecologies of the beetles and their morphological features.
In the end I think it went well. Here is the only photo from my presentation. It was taken by our Lab Technician Sarah Schmits. I didn't even made it to the water beetle people group picture... oh well...
The file with the presentation is posted at ResearchGate.
Thing is that I'm more interested and, so far, much more focused on morphology, which was only one of the three main themes I was supposed to talk about. I consider myself a lucky person in general. I've had all the support, data and information I've needed from my advisor, Dr. Andrew Short, our postdoc researcher, Dr. Emmanuel Toussaint and my labmates at the Short Lab. It was not easy to get smooth transitions between the molecular phylogeny that we have now, the different ecologies of the beetles and their morphological features.
In the end I think it went well. Here is the only photo from my presentation. It was taken by our Lab Technician Sarah Schmits. I didn't even made it to the water beetle people group picture... oh well...
The file with the presentation is posted at ResearchGate.
Wednesday, September 14, 2016
The amazing internal morphology
I've been doing full disarticulations of acidocerines, and so far, it has been fascinating!
For one thing, I wasn't sure about how to do it, but was pretty sure about what I wanted to see. I started trying to find a protocol for this, as I've seen this kind of work presented at national meetings, but maybe I didn't do a good job in my search, because I couldn't find one. And yes, I know the technique (mostly the KOH soft tissue dissolution) vary depending on several factors as the "age of preservation" of the specimen, its size and its hardness, but still I was looking for some kind of standard that I didn't find... at least not like that.
What I did found were several revisionary works on hydrophilids that include detailed morphology for the group in question. My favorite so far, in terms of the description of the methods, is by Minoshima, Komarek & Ôhara (2015) for the genus Megagraphydrus (currently Agraphydrus, which they synonymize in this work). Regarding morphological treatment and nomenclature of the head and its appendages, my favorite is Anton & Beutel for the genus Helophorus. Other useful resources are the revision of Afrotropical Anacaena by Komarek 2004 and the nice pictures provided by Fikáček & Vondráček (2014) on their revision of Pseudorygmodus.
My previous experiences with dissections were on broad-nosed weevils, of minimally 4mm which are harder (thicker cuticle) than the acidocerines, and my procedures involved nearly-boiling water and a quick "cooking" of about 10 minutes. The same treatment with water beetles can result in a disaster of two kinds: one, you overcook them in which case dissection is a very delicate and risky process where you end up tearing sclerites that you were not supposed to, or two, you undercook them, and then dissection is difficult and messy because there is soft tissue everywhere and you end up not being able to clearly see the structures under the microscope anyway. For my first experiments I used non-target specimens (I mean hydrophilids other than acidocerines from which we had a bunch sitting on vials on alcohol).
Then I made a patience commitment accompanied of deep breaths and performed the procedures as described in Minoshima et al (2015), which involve a temperature of around 60°C during about one hour. At the beginning I was afraid of overcooking but when I was able to relax and let it be, the results were quite good! I even tweeted it in excitement!!
I also have had to modify procedures, so now I separate head, prothorax and abdomen before cooking the specimen, also open the abdomen on one side (when possible), so the KOH goes through all the abdominal grease and tissues, and now I try to not cook the wings (something I should have done before... oh well).
So far I have 10 species fully dissected (one specimen each). There are interesting characters everywhere!: mouthparts (mandibles are particularly awesome!), metafurca, abdominal tergites and sternites, wings. And then of course, it helps a lot when you have a good microscope available to take a closer look!
For now, I will leave you with this mosaic of structures of Helochares abbreviatus. [structures not necessarily to scale regarding each other, images not edited -only cropped to fit slide-]
Disarticulation allows you to see structures that are usually overlooked because you cannot see them without breaking the specimen, such as the prosternal process for example. And then of course, as with some specimens you can't identify the sex, "by accident" you dissect a female and also find a bunch of structures that apparently not many hydrophilid workers take a look at. Again, this is only starting! the tricky next part is to start coding these things.
References
For one thing, I wasn't sure about how to do it, but was pretty sure about what I wanted to see. I started trying to find a protocol for this, as I've seen this kind of work presented at national meetings, but maybe I didn't do a good job in my search, because I couldn't find one. And yes, I know the technique (mostly the KOH soft tissue dissolution) vary depending on several factors as the "age of preservation" of the specimen, its size and its hardness, but still I was looking for some kind of standard that I didn't find... at least not like that.
What I did found were several revisionary works on hydrophilids that include detailed morphology for the group in question. My favorite so far, in terms of the description of the methods, is by Minoshima, Komarek & Ôhara (2015) for the genus Megagraphydrus (currently Agraphydrus, which they synonymize in this work). Regarding morphological treatment and nomenclature of the head and its appendages, my favorite is Anton & Beutel for the genus Helophorus. Other useful resources are the revision of Afrotropical Anacaena by Komarek 2004 and the nice pictures provided by Fikáček & Vondráček (2014) on their revision of Pseudorygmodus.
My previous experiences with dissections were on broad-nosed weevils, of minimally 4mm which are harder (thicker cuticle) than the acidocerines, and my procedures involved nearly-boiling water and a quick "cooking" of about 10 minutes. The same treatment with water beetles can result in a disaster of two kinds: one, you overcook them in which case dissection is a very delicate and risky process where you end up tearing sclerites that you were not supposed to, or two, you undercook them, and then dissection is difficult and messy because there is soft tissue everywhere and you end up not being able to clearly see the structures under the microscope anyway. For my first experiments I used non-target specimens (I mean hydrophilids other than acidocerines from which we had a bunch sitting on vials on alcohol).
Then I made a patience commitment accompanied of deep breaths and performed the procedures as described in Minoshima et al (2015), which involve a temperature of around 60°C during about one hour. At the beginning I was afraid of overcooking but when I was able to relax and let it be, the results were quite good! I even tweeted it in excitement!!
I also have had to modify procedures, so now I separate head, prothorax and abdomen before cooking the specimen, also open the abdomen on one side (when possible), so the KOH goes through all the abdominal grease and tissues, and now I try to not cook the wings (something I should have done before... oh well).
So far I have 10 species fully dissected (one specimen each). There are interesting characters everywhere!: mouthparts (mandibles are particularly awesome!), metafurca, abdominal tergites and sternites, wings. And then of course, it helps a lot when you have a good microscope available to take a closer look!
For now, I will leave you with this mosaic of structures of Helochares abbreviatus. [structures not necessarily to scale regarding each other, images not edited -only cropped to fit slide-]
Disarticulation allows you to see structures that are usually overlooked because you cannot see them without breaking the specimen, such as the prosternal process for example. And then of course, as with some specimens you can't identify the sex, "by accident" you dissect a female and also find a bunch of structures that apparently not many hydrophilid workers take a look at. Again, this is only starting! the tricky next part is to start coding these things.
References
- Anton, E., & Beutel, R. G. (2004). On the head morphology and systematic position of Helophorus (Coleoptera: Hydrophiloidea: Helophoridae). Zoologischer Anzeiger-A Journal of Comparative Zoology, 242(4), 313-346.
- Fikáček, M., & Vondráček, D. (2014). A review of Pseudorygmodus (Coleoptera: Hydrophilidae), with notes on the classification of the Anacaenini and on distribution of genera endemic to southern South America. Acta Entomologica Musei Nationalis Pragae, 54(2), 479-514.
- Komarek, A. Taxonomic revision of Anacaena Thomson, 1859 I. Afrotropical species. Koleopterologische Rundschau, 74, 303-349.
- Minoshima, Y. N., Komarek, A., & Ôhara, M. (2014). A revision of Megagraphydrus Hansen (Coleoptera, Hydrophilidae): synonymization with Agraphydrus Regimbart and description of seven new species. Zootaxa, 3930(1), 1-63.
Subscribe to:
Posts (Atom)



